Product description
Product Name: Detachable Electrochemical Cell Testing Device (e.g., Coin Cell, Swagelok-type)
Key Features and Specifications
| Feature Category | Specification |
| System Type | Reusable Electrochemical Test Fixture |
| Key Function | Secure and Leak-Proof Holder for two or three-electrode testing |
| Configuration | Detachable/Disassemblable design for easy electrode insertion and replacement |
| Material | Chemically Inert and Corrosion-Resistant (e.g., PTFE, PEEK, Stainless Steel) |
| Electrode Type | Compatible with various electrode formats (e.g., Coin, Plate, Disc, Membrane) |
| Reference Electrode | Dedicated port/chamber for a Reference Electrode (e.g., Ag/AgCl, Mercury Oxide) in three-electrode setup |
| Sealing | High-quality O-rings and Compression Seals for hermetic, leak-proof testing |
| Connectivity | Standardized connection ports (e.g., banana plugs) for simple interface with potentiostats/battery testers |
Product Summary
The Detachable Cell Testing Device is a crucial piece of Precision Instrumentation for electrochemists and battery researchers. It provides a robust, reusable, and easily assembled platform for conducting highly reliable and reproducible electrochemical measurements.
These devices, often utilizing a Swagelok-type or specialized compression design, allow researchers to quickly and accurately test the performance of new materials and half-cells in a controlled environment. The key benefit of the detachable design is the ease of disassembly and post-test analysis of the electrodes and separators, which is critical for understanding material degradation and failure mechanisms.
Primary Applications
New Energy Research:
Electrode Material Screening: Used for rapid, controlled testing of new anode, cathode, and solid-state electrolyte materials (e.g., Lithium, Sodium, Zinc-ion) in half-cell or full-cell configurations.
Electrolyte Optimization: Testing the stability and performance of various liquid, gel, or solid electrolytes.
Cycle and Rate Testing: Performing basic charge/discharge cycling, C-rate performance evaluation, and long-term stability studies.
Materials Chemistry & Functional Surfaces:
Electrocatalyst Testing: Employed to test the activity and stability of electrocatalysts (e.g., for HER, OER, CO₂ reduction) by allowing easy integration with working and counter electrodes.
Corrosion Studies: Used for setting up precise electrochemical cells for corrosion rate and material degradation analysis.
Advantages for Electrochemical Research
Reproducibility: The standardized, fixed internal geometry ensures consistent current distribution and accurate potential measurements, leading to highly reproducible data.
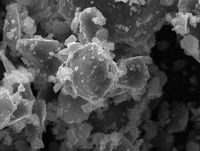
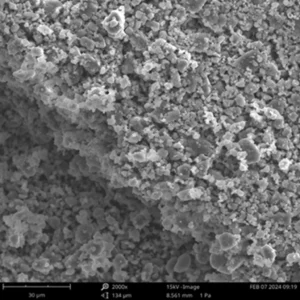
Post-Mortem Analysis: The easy-to-disassemble structure facilitates the recovery of electrodes and separators for detailed post-test characterization (e.g., SEM, XRD).
Versatility: Accommodates two-electrode (symmetric or full cell) and three-electrode (with reference electrode) configurations, offering comprehensive testing capabilities.
Corrosion Resistance: Constructed from high-purity, chemically inert materials to prevent contamination or degradation from aggressive electrolytes.